Abstract
Familial myelodysplastic syndromes (MDS) arise from haploinsufficiency of genes involved in hematopoiesis, show variable penetrance and are primarily associated with MDS in younger patients. The same genes involved in familial MDS might also cause sporadic manifestation, e.g. in GATA2 deficiency where ~2/3 of identified germline mutations arise de novo . In this study we aimed to discover the genetic cause in 7 patients of 4 unrelated pedigrees (Fig. A). The phenotypic denominator was MDS with progressive or transient loss of chromosome 7 and remarkably, spontaneous long-lasting remission was observed in 2 cases. Dysmorphic features or neurological symptoms were absent. Median age at diagnosis was 2 (1-42) years, all patients presented with thrombocytopenia with or without additional cytopenias and a hypocellular marrow without increase of blasts. There was no spontaneous growth in an in vitro GM-CSF hypersensitivity assay performed in 2 patients.
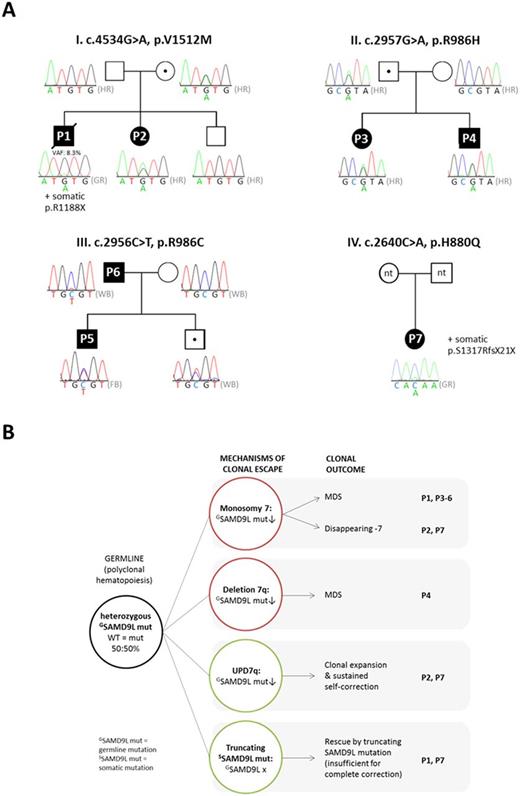
Genomic studies identified constitutional mutations p.H880Q, p.R986H, p.R986C and p.V1512M in SAMD9L gene on 7q21 cytoband, with decreased allele frequency in hematopoiesis. Interestingly, similar (p.I981T, p.C1196S) or identical (p.H880Q, p.R986C) SAMD9L mutations had been recently reported in 4 families with Ataxia-Pancytopenia syndrome. Family testing in 3 pedigrees (Fig A), revealed an incomplete penetrance in 33% (3/9) of mutation carriers. In comparison with other germline variants, SAMD9L missense mutations showed significantly lower median allelic frequencies in blood across all patients (53% vs. 16%, p<.05). The nonrandom loss of mutated SAMD9L allele was attained through monosomy 7, deletion 7q, uniparental isodisomy 7q, or acquired truncating SAMD9L variants p.R1188X and p.S1317RfsX21 (Fig. B). TA-cloning confirmed cis orientation of both SAMD9L mutations in P1. Additionally, acquired mutations were found in oncogenes in P1 at CMML disease stage (SETBP1 p .D868N, EZH2 p.V582M, KRAS p.Q61P) and in P5 (RUNX1 c.413_427+5dup20bp). Functional studies in cell lines confirmed growth-restrictive effect of SAMD9L mutations p.R986C and p.V1512M. Double-mutated SAMD9L p.V1512M-p.R1188X vector resulted in stable expression of a truncated protein lacking p.V1512M (suggesting a compensatory effect).
Leukemic progression from MDS to CMML was observed after 3.6 years in P1. In contrast, spontaneous remission occurred in P2 and P7, who showed transient monosomy 7 that had been replaced by large UPD7q clones with wildtype SAMD9L allelic duplication. Both patients maintained normal marrow morphology, cytogenetics, and blood counts until over 10 and 20 years from initial diagnosis.
In conclusion, our study establishes constitutional SAMD9L mutations as a novel cause of hereditary MDS without accessory phenotypes and point to the notion that MDS with chromosome 7 loss can be the sole and common manifestation of SAMD9L-related disease. The clinical outcomes range from leukemic progress to spontanous remission. This is the first observation of sustained revertant mosaicism due to UPD7q and etiologic explanation for transient monosomy 7. Further studies will be essential to define the role of SAMD9L in hematopoiesis and explore the observed variable penetrance.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal